how many valence electron does sulfur have|10.6: Valence Electrons : Bacolod The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. The elements that receive electrons and form bonds are called anions. During the formation of a bond, the last shell of sulfur receives two electrons and turns into a sulfide ion(S2 . Tingnan ang higit pa Watch chupaeng on SpankBang now! - Pinay, Pinayflix, Teenager Porn - SpankBangThe completely dark reels make the symbols really pop – up. It would be foolish not to expect mariachi music as the soundtrack in this case. Upbeat and loud, guitars and trumpets make the whole gameplay .
PH0 · Valences of the Chemical Elements
PH1 · Valence electrons (video)
PH2 · Valence Electrons Chart for All Elements
PH3 · How to find the Valence Electrons for Sulfur (S)
PH4 · How to Find the Valence Electrons for Sulfur (S)?
PH5 · How many valence electrons does sulfur have?
PH6 · How many valence electrons are in an atom of sulfur?
PH7 · How Many Valence Electrons Does Sulfur (S) Have?
PH8 · Determine valence electrons using the periodic table
PH9 · 2.7: Applications of Electron Configurations: Valence
PH10 · 10.6: Valence Electrons
how many valence electron does sulfur have*******The elements that have 5, 6, or 7 electrons in the last shell receive the electrons in the last shell during bond formation. The elements that receive electrons and form bonds are called anions. During the formation of a bond, the last shell of sulfur receives two electrons and turns into a sulfide ion(S2 . Tingnan ang higit paThe second element in group-16 is sulfur(S). The valence electron is the total number of electrons in the last orbit. The total number of electrons in the last shell(orbit) . Tingnan ang higit pa
The valence electron has to be determined by following a few steps. The electron configuration is one of them. It is not possible to . Tingnan ang higit paSulfur participates in the formation of bonds through its valence electrons. This valence electron participates in the formation of bonds with atoms of other elements. . Tingnan ang higit paThe ability of one atom of an element to join another atom during the formation of a molecule is called valency(valence). There are some . Tingnan ang higit pa Mar 23, 2023 There are two ways to find the number of valence electrons in Sulfur (S). The first is to use the Periodic Table to figure out how many electrons Sulfur has in its . Sulfur has six valence electrons. Valence electrons are the outermost electrons which, therefore, are located on the highest energy levels. Consequently, .
how many valence electron does sulfur have 10.6: Valence Electrons Example \(\PageIndex{1}\): Number of Valence Electrons. How many valence electrons are in one atom of each element? sulfur; helium; potassium; . May 19, 2024
The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different .
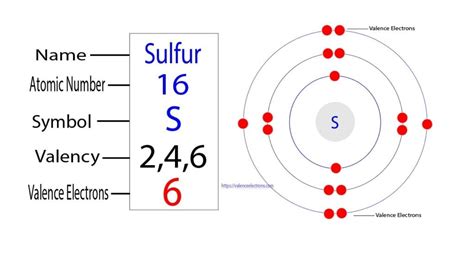
Consider sulfur's electron configuration, which was determined in the previous section and is replicated below. 1s22s22p63s23p4. Recall that the energy levels in an electron . Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as . Sulfur's Valence Electrons: The Key to Chemical Behavior • Sulfur's Valence Electrons • Discover why sulfur's six valence electrons are crucial for its chemical . This table of element valences includes the maximum valence and most common valence values in chemistry. . (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Remember an element's electron cloud will become more stable by filling, emptying, or half-filling the .how many valence electron does sulfur have The valency of sulfur is actually very subjective since it varies. In a few situations, sulfur may have the valency of two while in other cases it may be six. Sulfur basically holds six electrons in its outer shell. In the general scenario, it’s easier for the sulfur to gain 2 electrons rather than losing six.Sulfur. 16. 32.06. Glossary. GroupA vertical column in the periodic table. Members of a group typically have similar properties and electron configurations in their outer shell. PeriodA horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right. Ions are atoms/molecules with a non-neutral net charge because it gave or took an electron. Ionic compounds are elements bonded together through electron-giving. In other .An atom's valence electrons are the electrons in its outermost shell. In the chlorine model below, the valence electrons are shown in red . The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can .
Note that the third shell can contain 2(3)2 =18 electrons in this case, but the sulphur atom only has six electrons, so the last shell contains 6 electrons. 4. Valence Electrons in Sulphur. Valence electrons are electrons that are distributed in the atom’s outermost shell. Sulfur is in the third period and is a member of the Oxygen family . Sulfur has six valence electrons, meaning that each atom of this element has six electrons in its outermost shell. The number of valence electrons that each element has can be predicted based on its location on the periodic table, though this only applies to neutral atoms. An element's main group number indicates how many valence .Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons .The atomic number of Sulfur S is 16. The electronic configuration of Sulfur S can be written as 1 s 2 2 s 2 2 p 6 3 s 2 3 p 4; The valence electrons are the sum of the electrons in the outermost shell, that is two 3 s electrons and four 3 p electrons which gives a total of six valence electrons. Therefore, the valence electron in a Sulfur S . Sulfur's Valence Electrons: The Key to Chemical Behavior • Sulfur's Valence Electrons • Discover why sulfur's six valence electrons are crucial for its chemi. To determine the valence electrons of SO 3, it is first necessary to know the valence electrons of the oxygen and sulfur atoms. To determine the valence electrons of sulfur trioxide we have to follow .10.6: Valence Electrons For atoms with many electrons, this notation can become lengthy and so an abbreviated notation is used. The electron configuration can be visualized as the core electrons, equivalent to the noble gas of the . To determine the valence electrons of SF 6, it is first necessary to know the valence electrons of the fluorine and sulfur atoms. To determine the valence electrons of sulfur hexafluoride we have to .And so for this video, we're only talking about the valence electrons for elements in the main groups. When we talk about the main groups, you're using the one through eight system for classifying groups. So one, two, three, four, five, six, seven, and eight. So we're going to ignore the other way to number the groups.The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining four electrons. Therefore the sulfur electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 4.
How many valence electrons does Sulfur? there are 6 electrons in valence shell of sulphur so it accepts two electrons to complete the octet (8 electrons in last shell) so its valency in ionic .
Sulfur has 6 Valence electrons, 2 in the first shell, 8 in the second shell, and 6 in the outermost layer (third layer). They can determine the number of kernel electrons and the number of valence electrons due to the bonds they form, for example Sulfur is more likely to form ions with the Alkaline earth metals and form different covalent bonds.

Every subshell has a # of orbits s/p/d/f that can each hold 2 electrons each (one has the opposite spin of the other). The first shell (of all atoms) has 1 subshell of s-orbitals containing 1 s orbital. This means that the first shell can hold 2 electrons. The second shell has 2 subshells: 1 s-orbital and 3 p-orbitals.
Disclaimer: Mangyaring tandaan na ang aming Aquarius (Kumbh Rashi) Ang mga masuwerteng numero ng lottery ay hindi kasama ng garantiya ng isang panalo sa lottery.Tandaan na the lottery ay isang laro ng pagkakataon at isang ganap na randomized na proseso, na walang tiyak na paraan upang malaman kung aling mga numero ang .
how many valence electron does sulfur have|10.6: Valence Electrons